FDA Greenlights Merck’s Enflonsia to Shield Infants from RSV
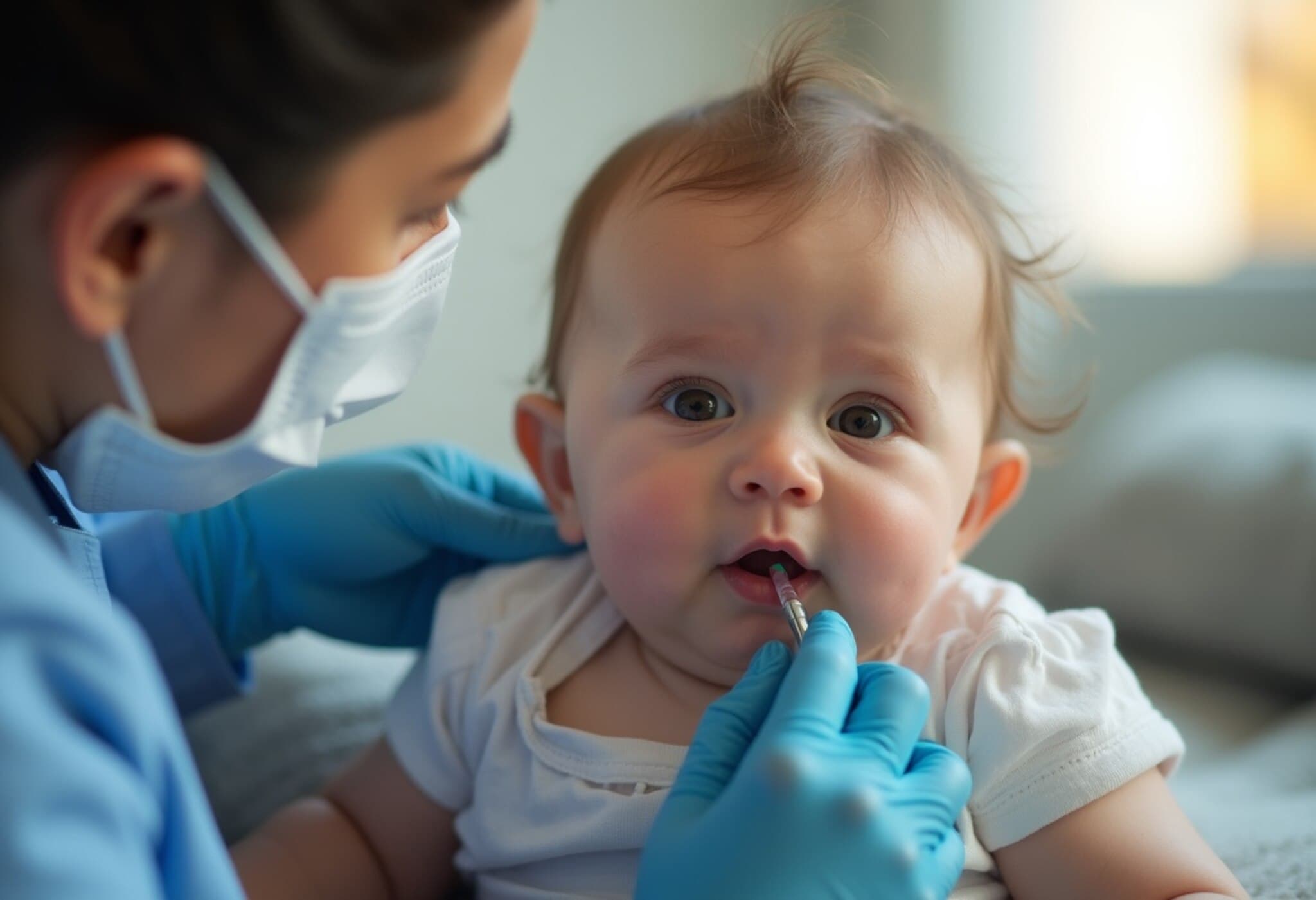
The Food and Drug Administration has given the nod to Merck’s new respiratory syncytial virus (RSV) antibody shot, Enflonsia, designed to protect infants during their first RSV season. This approval allows Merck to release the drug in time for the upcoming RSV season, which usually hits in fall and winter and extends into spring.
What This Means for Parents and Healthcare Providers
Merck expects to begin taking orders as early as July, aiming to deliver doses before RSV widely spreads. RSV remains a serious threat, causing thousands of deaths in older adults and hundreds in infants annually, with complications being the leading reason for hospitalizations among newborns.
Dr. Dean Li, president of Merck Research Laboratories, emphasized the company’s commitment to making Enflonsia accessible ahead of the season to ease the burden of this widespread infection on families and healthcare systems.
Competition Intensifies: Enflonsia vs. Beyfortus
Enflonsia enters a market where it will face direct competition from Beyfortus, a similar monoclonal antibody treatment developed by Sanofi and AstraZeneca. Beyfortus experienced nationwide shortages during last year’s unprecedented demand surge.
While both drugs provide immediate protection by delivering antibodies directly into the bloodstream, they target different parts of the RSV virus, making direct comparisons complex. One notable advantage of Enflonsia is its administration independent of infant weight, potentially simplifying dosing. Beyfortus requires dosing adjusted by the infant’s body weight.
Market Dynamics and Supply Updates
Sanofi has announced efforts to boost Beyfortus supplies, including plans to begin early third-quarter shipments. The drug generated sales of approximately €1.7 billion (around $1.8 billion) last year, reflecting its blockbuster status.
Overview of RSV Prevention Options
Beyond monoclonal antibodies, RSV vaccines exist for adults and pregnant women but are not approved for young children. Recently, the FDA paused approvals for RSV shots in young children amid ongoing safety evaluations.
All manufacturers are now awaiting a critical meeting of external vaccine advisors to the Centers for Disease Control and Prevention, scheduled from June 25 to 27. This panel will recommend immunization policies concerning RSV and related vaccines.
Clinical Trial Success
Mid- to late-stage trials for Enflonsia revealed promising results: it led to an 84% reduction in RSV-related hospitalizations and a 90% decrease in hospitalizations due to lower respiratory infections in infants up to five months old compared with placebo. Additionally, the shot cut lower respiratory infections requiring medical care by over 60% during this period.
Why RSV Protection Matters
RSV is a common cause of lower respiratory infections such as pneumonia, especially threatening to infants and older adults. New preventive measures, like Enflonsia, represent critical advancements in reducing these infections and their associated hospitalizations.
Stay tuned for further updates as this new treatment rolls out and the CDC formulates its immunization guidelines.