CDC Vaccine Advisory Panel Greenlights Merck’s RSV Injection for Infants
The Advisory Committee on Immunization Practices (ACIP) has officially recommended Merck’s new shot designed to protect infants from respiratory syncytial virus (RSV) during their first season. This endorsement marks a significant step for public health officials and vaccine manufacturers alike.
Unanimous Support Amid Leadership Changes
Under the leadership of Health and Human Services Secretary Robert F. Kennedy Jr., the ACIP was recently overhauled, stirring concern in the medical community due to the inclusion of outspoken vaccine critics. Despite these changes, the panel voted unanimously to recommend Merck’s RSV preventive treatment, known as Enflonsia, and approved its inclusion in a federal vaccine program that guarantees free access for eligible children.
A Timely Approval Ahead of RSV Season
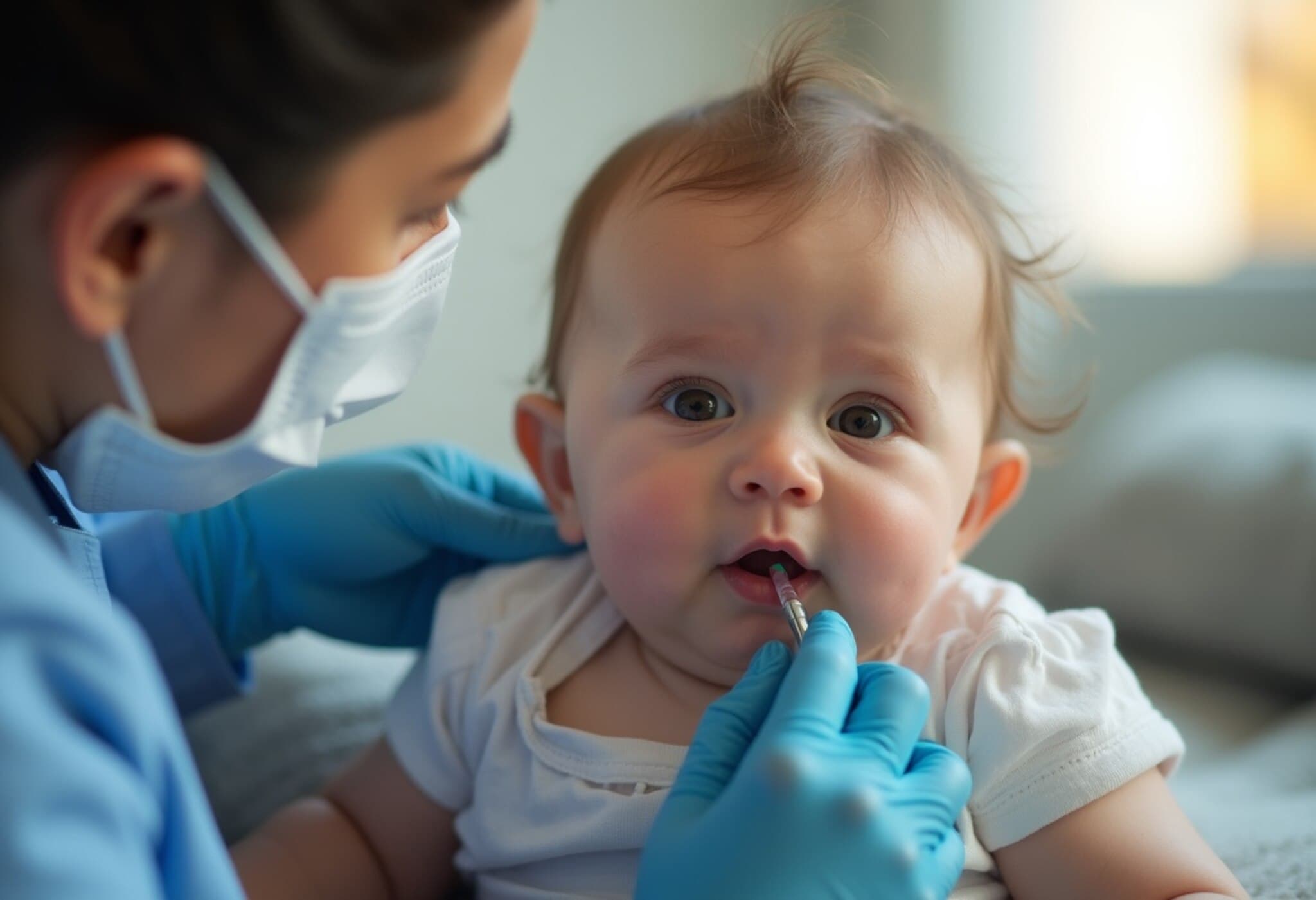
This approval allows Merck to introduce Enflonsia just before the RSV season begins in fall and continues through spring, ensuring infants can receive protection early. Enflonsia, administered during an infant’s first RSV season, will enter a competitive market dominated by another monoclonal antibody injection called Beyfortus, offered jointly by AstraZeneca and Sanofi.
How Enflonsia Works
Both Enflonsia and Beyfortus are monoclonal antibodies that provide immediate immunity by delivering antibodies directly into the bloodstream. Although they target different sites on the virus, each is designed to significantly reduce RSV infection risks during infancy — a period when infants face the highest risk of complications.
The Stakes: RSV’s Threat to Infants and Seniors
RSV remains a leading cause of hospitalization for newborns and causes thousands of deaths each year among older adults and hundreds of infant fatalities. Recent clinical trials of Enflonsia demonstrated remarkable effectiveness, reducing RSV-related hospital stays by over 84% and hospitalizations due to lower respiratory tract infections by 90% compared to placebo groups in infants up to five months old.
Safety Debates and Expert Endorsements
Although the panel included two members known for vaccine skepticism, Retsef Levi and Vicky Pebsworth, who voted against the shot citing safety concerns, the majority highlighted the robust data backing Enflonsia’s safety and efficacy. The Food and Drug Administration granted the injection approval just weeks earlier.
Dr. Cody Meissner, a pediatric expert with advisory experience at both CDC and FDA, emphasized, "These products are safe and effective, with no additional data needed." Echoing this view, Dr. Jason Goldman, president of the American College of Physicians, called the development “a tremendous advance for medical science” and urged swift committee approval.
Targeted Recommendation for Infant Vaccination
The ACIP’s final recommendation specifies administering a single dose of Merck’s RSV shot to infants aged 8 months or younger, particularly for those born during or heading into their first RSV season. This targeted approach aims to maximize protection when infants are most vulnerable.
Looking Ahead
With this endorsement, Merck is set to expand the tools available to combat RSV, bolstering infant health protections during a critical period. While some voices call for caution, the consensus among health experts affirms the shot’s potential to save lives and reduce hospitalizations significantly.