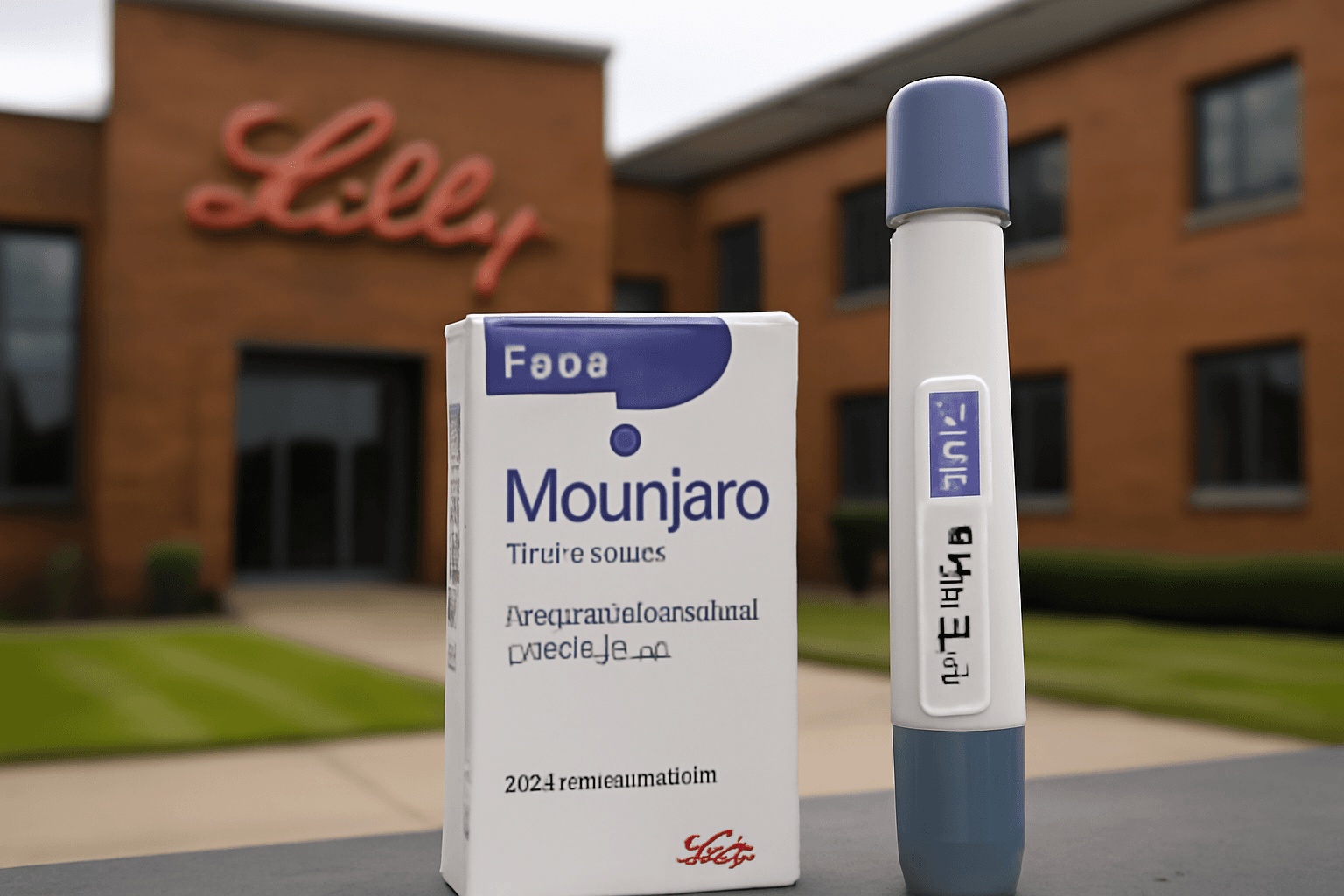
Eli Lilly’s Oral Obesity Pill Demonstrates Significant Weight Loss in Late-Stage Trial
Eli Lilly recently unveiled promising results from its late-stage clinical trial of orforglipron, a novel oral obesity medication. After 72 weeks, participants taking the highest dose experienced an average weight loss of nearly 12%, amounting to roughly 27 pounds. These findings push the pill closer to regulatory submission and possible approval as a groundbreaking, needle-free alternative in the thriving market for GLP-1 based weight loss drugs.
Weight Loss Impact and Market Expectations
The trial highlighted that 59% of patients on the highest dose lost at least 10% of their body weight, while more than 39% shed at least 15%. Although these numbers are compelling, they slightly underperformed some Wall Street analysts’ expectations of a 15% average weight reduction. Additionally, the results are comparable, yet modestly lower than the benchmark set by Eli Lilly’s own injectable blockbuster obesity drug, Wegovy.
The release of this data triggered a more than 13% drop in Eli Lilly’s shares during premarket trading, reflecting investor caution. This reaction signals the delicate balance between exciting medical advances and high market expectations in the rapidly expanding weight loss sector.
Expert Perspectives: Promise Meets Practical Concerns
Medical experts recognize the milestone represented by a highly effective oral GLP-1 option, especially for patients averse to injections. Dr. Jaime Almandoz, Medical Director of the Weight Wellness Program at UT Southwestern Medical Center, described the weight loss as "a significant and clinically meaningful outcome" and stressed how an oral agent could transform obesity care by improving accessibility.
Dr. Mihail "Misha" Zilbermint from Johns Hopkins Community Physicians called the pill a potential "game changer" pending tolerability once broadly prescribed. However, other clinicians raised concerns about patient adherence. Side effects, primarily gastrointestinal like nausea and vomiting, caused about 10.3% of patients on the highest dose to discontinue treatment — noticeably higher than with injectable counterparts.
Side Effects and Treatment Adherence Challenge
The trial reported gastrointestinal side effects affected up to a third of participants on the highest dose, with 24% experiencing vomiting, 33.7% nausea, and 23.1% diarrhea. These adverse reactions contributed to discontinuation rates that some experts argue could temper enthusiasm. Dr. Caroline Apovian from Brigham and Women’s Hospital highlighted the need for cautious optimism, emphasizing that excitement around new pills can sometimes outpace real-world tolerability.
Regulatory Pathway and Market Impact
Eli Lilly plans to submit the orforglipron data to regulators by the end of 2025, anticipating a commercial launch in 2026. Ken Custer, President of Lilly Cardiometabolic Health, emphasized the capacity of an oral pill to expand patient access dramatically—potentially addressing the unmet need for tens of millions more patients beyond the current 8 million on injectables.
This oral option also promises to simplify manufacturing and distribution, bypassing the complex supply chains and administration challenges associated with injectables. As such, orforglipron could fortify Eli Lilly’s market position against competitors like Novo Nordisk, which dominates with injectable treatments and has its own oral diabetes pill, Rybelsus.
Economic Considerations and Insurance Landscape
Cost remains a significant barrier. Current injectable GLP-1 drugs can cost close to $1,000 per month before insurance. Dr. Amy Sheer from the University of Florida hopes the oral pill will be more affordable since it avoids the expensive injection devices, potentially encouraging broader insurance coverage and improved patient access.
Scientific Insights and Upcoming Data
Unlike peptide-based GLP-1 injectables and Rybelsus, orforglipron is a non-peptide molecule, offering improved absorption and fewer dietary restrictions. This innovation could redefine convenience for patients managing obesity or type 2 diabetes.
Additional phase three data, including studies focused on patients with obesity and type 2 diabetes, will be presented at a major European medical conference in September and published in peer-reviewed journals later this year.
Looking Ahead: The Future of Oral GLP-1 Therapies
Industry analysts forecast the GLP-1 market to surpass $150 billion annually by the early 2030s, with oral agents potentially reaching $50 billion. Eli Lilly currently leads by about three years in oral GLP-1 development ahead of rivals like Novo Nordisk and others in the pipeline.
As this oral pill moves toward market entry, it raises important questions about balancing efficacy, tolerability, patient preferences, and cost. How clinicians and patients weigh these factors will shape the treatment landscape in obesity — a condition affecting millions and linked to numerous chronic diseases.
Editor’s Note
Orforglipron’s trial results mark a pivotal moment in obesity treatment by potentially offering a convenient, needle-free alternative that expands patient choice. However, managing side effects and ensuring adherence will be critical to realizing its promise. As the healthcare system grapples with rising obesity rates, innovations like this pill highlight the ongoing evolution of therapies balancing efficacy and user-friendliness. The coming regulatory reviews and real-world data will be crucial to understanding whether this new oral option can fulfill its transformative potential.